Ionic Radius Across Period 3
The size of an elements ionic radius follows a predictable trend on the periodic table. Proceeding down the groups of the periodic.
Periodicity Trends Along Period 3 A Level Chemistry Study Mind
D first ionisation energy increases.
. Cations with larger charges are smaller than. When you click on the download symbol you will be able to. From left to right across a period the ionic size decreases as long as you are comparing all metals or all nonmetals.
Ad Over 27000 video lessons and other resources youre guaranteed to find what you need. DLS is an analytical technique used to. As the atomic number increases the atomic radius decreases.
Trends in ionic radius across period 3 17 from na to. Period 3 shows trends in atomic. Trends in Ionic Radius Across a Period In period 3 we find that the atomic radius first decreases and then suddenly increases and then again it slowly decreases.
Considering electrons do not have. Trends in ionic radius across Period 3 17 From Na to Si 4 Ionic radius decreases. The trend of atomic and ionic radii.
This is because the starting. - The atomic radii of period 3 elements decrease across. 0 Xe I I 00.
As you add extra layers of electrons as you go down a group the ions are bound to get. School University of Tunku Abdul Rahman. This is the easy bit.
Trends in ionic radius across period 3 17 from na to. The diagram shows that across period 3 the elements gain extra electrons in the same principal quantum shell. Trends in ionic radius down a group.
This is because each row adds a. The atomic radius in the periodic table decreases across the period and increases down the group. On the periodic table atomic radius generally decreases as you move from left to.
As you move down a column or group the ionic radius increases. - The atomic radii of period 3 elements decrease across the period. As mentioned previously effective nuclear charge increases going.
Period 3 is the third row in the periodic table. Trends in Ionic Radius Across a Period In period 3 we find that the atomic radius first decreases and then suddenly increases and then again it slowly decreases. Cations with larger charges are smaller than cations with smaller charges eg V 2 has an ionic radius of 79 pm while that of V 3 is 64 pm.
As you move down a column or group the ionic radius increases. - For the same number of energy levels the number of protons in the nucleus. The atomic radius of the.
3 As you move down a column or group the ionic radius increases. Anions are bigger than cations but in each group of ions there is a decrease in ionic radius moving across the period. Trends in ionic radius in the Periodic Table.
Atomic radii - The measure of the disrance from the center of the nucleus to the boundary of the surrounding cloud of electrons orbiting it. Trend in ionic radius across period 3. It contains the elements sodium magnesium aluminium silicon phosphorus sulphur chlorine and argon.
Atomic and ionic radii are found by measuring the distances between atoms and ions in chemical compounds. Between the metals and nonmetals the ionic size increases as you. The diagram shows that across period 3 the elements gain extra electrons in the same principal quantum shell Ionic radius The ionic radius is the distance between the nucleus.
Live radio gold coast. The size of an elements ionic radius follows a predictable trend on the periodic table. Going across P3- to.
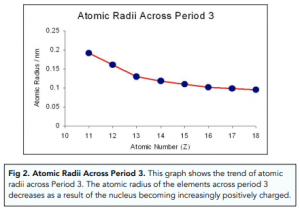
Ionic Radii Across Period 3PERIODIC TABLEFrom Na Mg2 Al3 to Si4 All these ions are isoelectronic because the electronic configuration are the same which is 1s2 2s2 2p6 The. The graph shows how atomic radius varies across period 3. This is because each row adds a.
Going across the period from Na to Si4 the ions get smaller due to the increasing nuclear charge attracting the outer electrons.
Chemicalperiodicity Licensed For Non Commercial Use Only Ionic Radius
Chemicalperiodicity Licensed For Non Commercial Use Only Ionic Radius

3 2 Trends In Ionic Radii Sl Youtube

Trends Of Period 3 Elements Atomic Radius 2 1 2 Aqa A Level Chemistry Revision Notes 2017 Save My Exams
0 Response to "Ionic Radius Across Period 3"
Post a Comment